Overview of Research Focus
The creation of macromolecular systems with precise control over their structural and functional properties constitutes a fundamental challenge for molecular engineering. Development of the ability to construct complex biomolecular architectures will provide a solution to this challenge. My research group has been keen on a unique synthetic biology approach to advanced biomolecular architectures and soft materials with “living” features .
The central idea is to use artificial genes to direct microbes to synthesize proteins or protein-like macromolecules, followed by controlled assembly of these biomolecules or even living cells into higher-order structures under mild physiological conditions, without the need for chemical modification. This approach has enabled not only the faithful transfer of the functions at the molecular level to the material properties at the macroscopic level — which used to be a fundamental challenge facing the bottom-up approach for material design, but also some unexpected emergent properties often associated with out-of-equilibrium complex systems.
In addition, the controlled assembly of protein molecules has also provided us with some exciting tools for studying protein phase separation, particularly in neurons—an important biological phenomenon with wide-ranging impact on biological regulation and disease pathology. Along this process, we have also enriched the arsenal of optogenetics by introducing some new motifs, such as cobalamin- or chlorophyll-binding proteins, which are prevalent in nature but rarely used in biotechnologies. The resulting materials and optogenetic tools hold great potential for a variety of applications ranging from regenerative medicine to neuroscience, ocean biotechnology and environmental remediation.
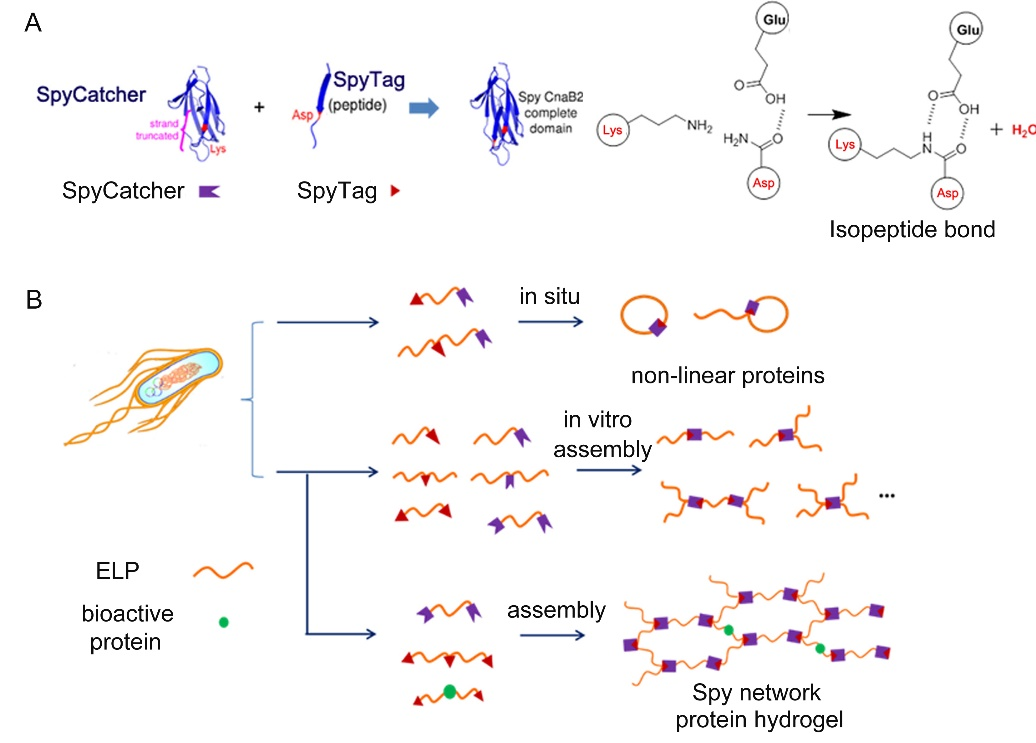
Genetically Encoded Click Chemistry
The past few years have witnessed the emergence of a new category of peptide/protein chemistry, such as SpyTag/SpyCatcher and SnoopTag/SnoopCatcher chemistry, which can covalently stitch together protein/peptide molecules with high specificity under mild physiological conditions. It has prompted the conceptualization of genetically encoded click chemistry (GECC) to describe a family of “ideal” peptide-protein reactive pairs possessing essential features of “click” chemistry while being also genetically encodable[1]. We envisioned that by bridging synthetic biology and materials science, a GECC toolbox comprising multiple mutually orthogonal, covalent-bond forming peptide/protein reactive pairs would open the door to new materials. We have elaborated on the use of GECCs for material design in our Review papers (Chin. Chem. Lett. 2017, 28, 2078), which has won “Excellent Paper Award” in 2018, and (Chin. J. Chem. 2020, 38, 894).
Using SpyTag/SpyCatcher chemistry, a prototype of GECC, we have previously demonstrated cellular synthesis of cyclic and tadpole-shaped elastin-like proteins (ELPs) as well as in vitro assembly of ELPs into other non-linear topologies such as block, 3-arm star, 4-arm star and H-shaped proteins (Figure 1) [2]. This study has for the first time proven the feasibility of protein topology engineering. This work has been highlighted by JACS Spotlights, C&E News and Faculty of 1000, with positive comments like "open the door to a multitude of new protein-based architectures" and "Good for teaching; technical advance".
We subsequently demonstrated the synthesis of an entirely recombinant protein-based hydrogel, namely the “Spy network”, which can further be designed to contain multiple bioactive motifs such as RGD cell binding domains, matrix metalloproteinase (MMP) cleavage sites, and even leukemia inhibitory factors (LIFs) [3]. The resulting LIF-Spy network hydrogel has been shown to be able to support 3D culturing of mouse embryonic stem cells (mESCs). These Spy network hydrogels demonstrated the feasibility of directly assembling recombinant protein molecules into macroscopic materials without the need for chemical modification while maintaining the molecular functions within the materials’ scaffolds. This work has been published in PNAS (Proc. Natl. Acad. Sci. USA 2014, 111, 11269-11274). Prof. Mark Howarth (Oxford) strongly recommended this study as “of outstanding interest” in his review article (Current Opinion in Chemical Biology 2015, 29, 94).
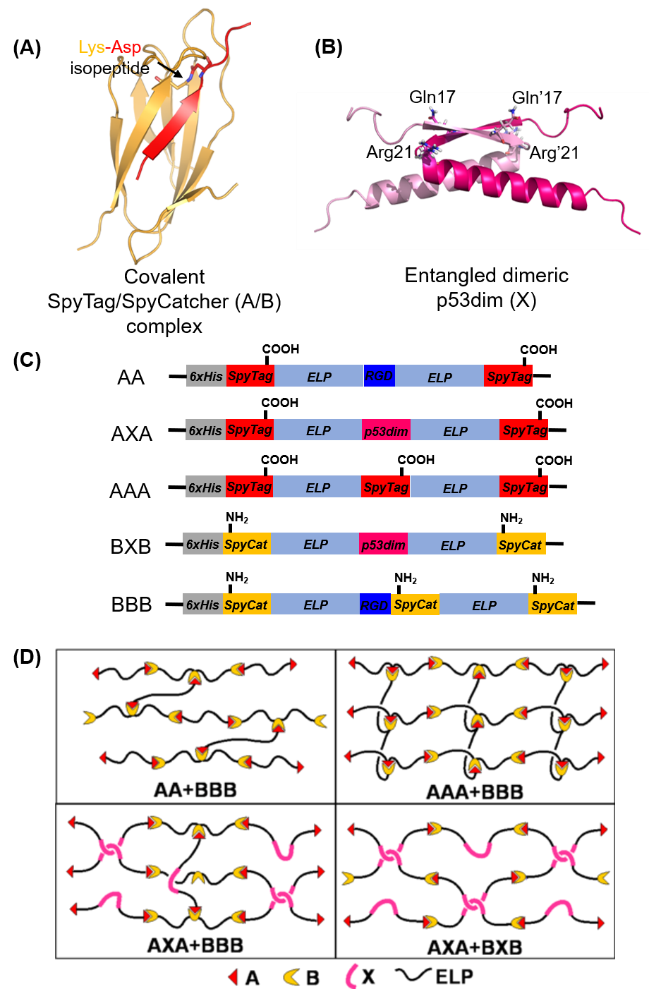
Stress relaxation, a hallmark of viscoelastic materials, has profound influence on cell-materials interactions. Drawing on covalent-bond forming GECC, mechanically entangled p53 dimerization and site-directed mutagenesis, we have synthesized a series of elastin-like polypeptide (ELP)-based hydrogels that contain abundant RGD cell-binding ligands but differ in their viscoelastic properties including stress-relaxation behavior (Figure 2). Since the stress-relaxation behavior of these protein networks at the macroscopic level is directly linked to protein-protein interactions at the molecular level, the dissipation modes of these networks revealed by rheological studies provided new insights into the kinetics and mechanical stability of protein-protein interactions—to obtain such knowledge requires single-molecule force spectroscopy otherwise. The delicate control we have over the structural and functional properties of these molecular networks opens up a new way to study cell-matrix interactions. This study has been published in ACS Macro Letter [4].
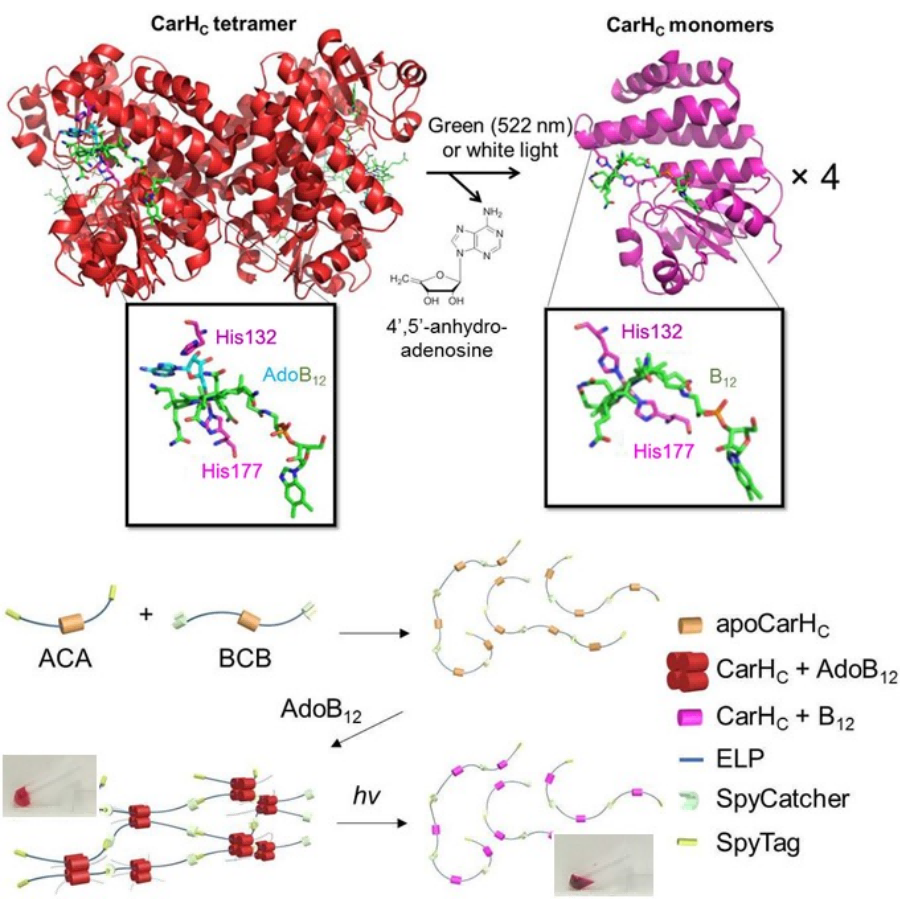
Photoresponsive Protein Materials Enabled by GECC
Dynamic, stimuli-responsive biomaterials hold great promise for stem cell regulation as well as therapeutic delivery. Given the great diversity of stimuli-responsive proteins in nature, the direct assembly of these proteins into macroscopic materials would provide a versatile approach for developing “smart” materials. The challenge lies in how to transfer the molecular functions to the resulting material properties through the polymerization/assembly process. Taking advantage of GECCs, my research group has recently created B12-dependent photo-responsive, entirely protein-based hydrogels by covalently polymerizing light-sensitive CarH proteins [5]. The CarH protein is an AdoB12-binding light-dependent transcriptional regulator that controls carotenoid biosynthesis in bacterial cells [6-9]. A unique feature of CarH is that its C-terminal domains (CarHC) form a tetramer when binding to the cofactor AdoB12 under dark conditions and disassemble into monomers accompanied with the photolysis of AdoB12 on exposure to green light (512 nm) or white light. CarHC polymers that were synthesized simply by mixing telechelic SpyTag-ELP-CarHC-ELP-SpyTag (ACA) and SpyCatcher-ELP-CarHC-ELP-SpyCatcher (BCB) at an equimolar ratio form a hydrogel in the presence of AdoB12 in the dark and underwent a rapid gel-sol transition upon exposure to light (Figure 4). Such a light-dependent gel-sol transition was utilized for controlled release of stem cells and protein molecules [5]. This study has been published in PNAS (Wang et al., Proc. Natl. Acad. Sci. U S A. 2017, 114, 5912) and has received massive media attention. The paper was selected as the Hong Kong Institution of Engineers Best Materials Paper in 2017. Prof. Mark Howarth (Oxford) highly commended this work as “of special interest” and “innovative development of chemical and light-triggered protein/protein interactions” in his Review article (Curr. Opin. Biotech. 2018, 51, 16).
This entirely protein-based photoresponsive hydrogel system has proven to be a robust 3D cell culture system that enables harmless transfer (encapsulation and release) of cells. We have been granted with 1 US patent and have founded SPES Tech Limited, a Hong Kong-based biotech startup company with focus on synthetic biology approaches for designing engineered protein materials. The company has now been part of Merck Accelerator Program and has received multiple endowments from Merck, the Incu-Bio Program of Hong Kong Science Park, Tsinghua Research Institute in Guangzhou, the Innovation and Technology Commission of Hong Kong, and HKUST U*STAR Program.
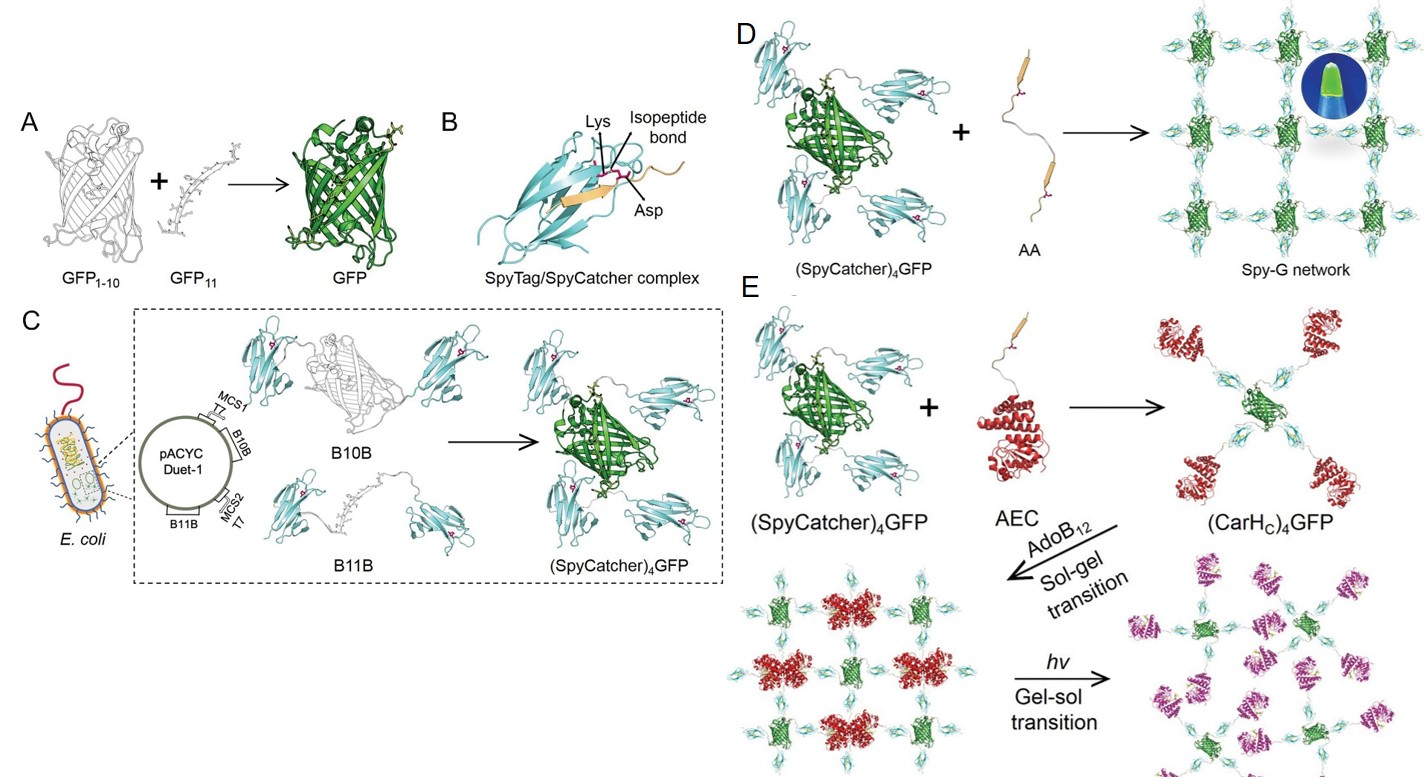
Dynamically Tunable Protein Materials Enabled by Protein Topology Engineering
As aforementioned, design of proteins with nonlinear topologies is a nascent branch of protein engineering, but of which significant applications remain to be seen. My research group has recently demonstrated the cellular synthesis of (SpyCatcher)4GFP, a 4-arm star-like protein enabled by spontaneous split-GFP reconstitution, which further led to the creation of various protein networks exhibiting tunable mechanics and suitability for cell encapsulation (Figure 4). A derivative 4-arm star-like protein, (CarHC)4GFP, resulting from the conjugation of (SpyCatcher)4GFP with the SpyTag-fusion CarHC photoreceptors, can undergo rapid sol-gel and gel-sol transitions in response to AdoB12 and light, respectively. The chemo- and photo-induced phase transitions enabled encapsulation and controlled release of protein molecules such as the biofilm-degrading glycosyl hydrolase PslG, a potential agent for combatting those multidrug-resistant bacterial species in chronic infections. This study illustrates protein topology engineering as a new strategy for designing dynamically tunable biomaterials. This work has been published in Matter, the flagship journal in materials science owned by Cell Press (Yang et al., Matter 2020, 2, 233). One reviewer commended that “this work is innovative and highly interdisciplinary and provides a new way of thinking for protein engineering and materials science”.
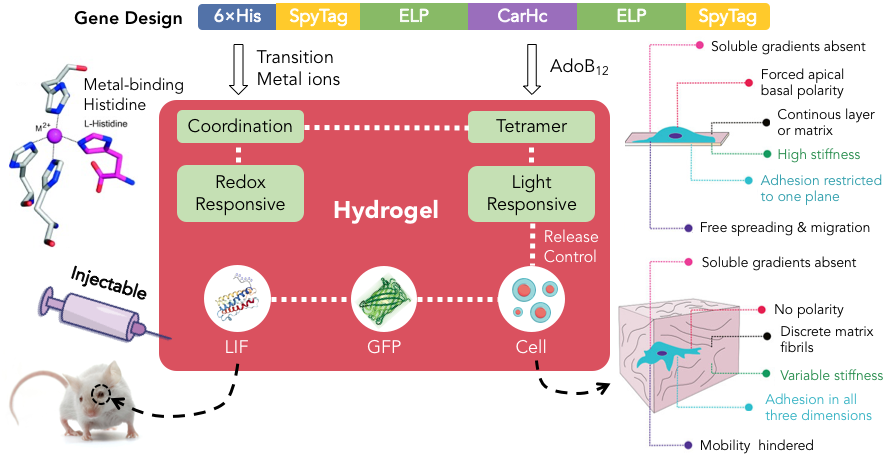
Injectable Smart Materials Enabled by Metal-Directed Protein Assembly
In addition to using GECC, we also used metal-directed assembly for creating injectable protein materials[11] [12]. Because of the reversible and dynamic nature of metal-ligand interactions, the resulting materials often exhibited marked mechanical properties such as high stiffness and robust self-healing, as well as redox-responsiveness and injectability.
One of our studies showed that the complexes of CarHC and transition metal ions (Co, Ni, Zn or Cu) underwent a rapid sol-gel transition upon addition of AdoB12, leading to the formation of hydrogels that displayed marked self-healing, injectability and photo-degradability (Figure 5). The inducible phase transitions of these materials further enabled facile encapsulation and release of cells and proteins. Injecting the Zn2+-coordinated gels decorated with leukemia inhibitory factor (LIF) into the injury sites of mouse optic nerves led to prolonged cellular signaling, enhanced axon regeneration and improved neuronal survival. We successfully demonstrated a simple yet powerful strategy for designing injectable protein materials for regenerative neurobiology (Jiang et al., Science Advances 2020, 6, eabc4824).
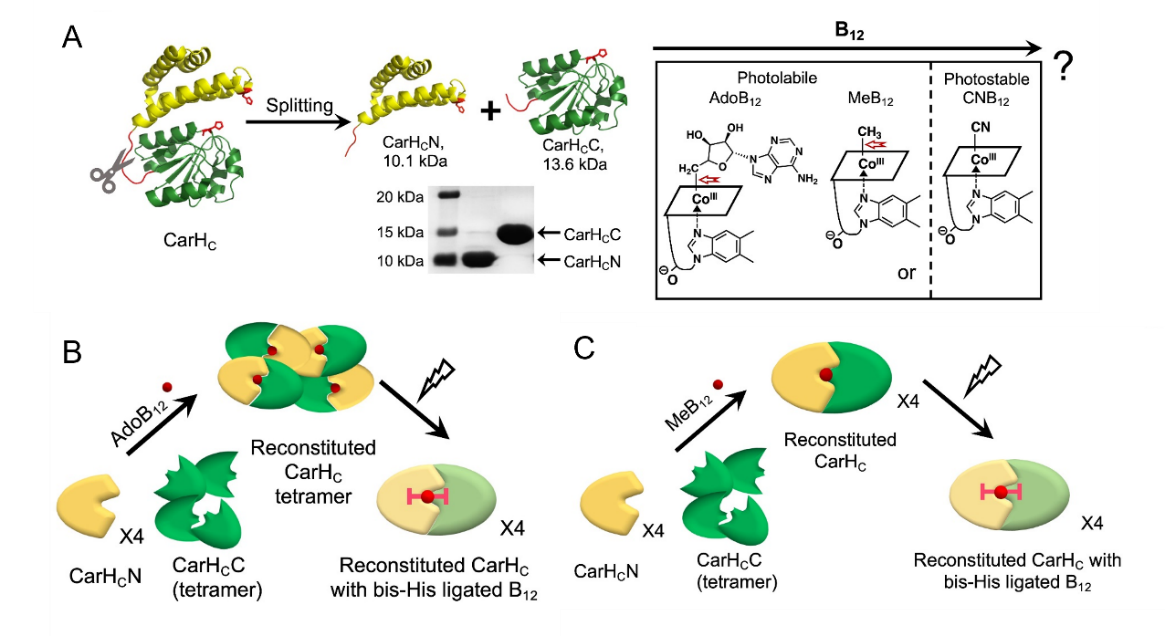
Split Photoreceptor Protein as an Alternative GECC
Despite their great potential in biomedical research and molecular engineering, molecular tools based on inducible split-protein reconstitution, chemically inducible dimerization in particular, are often plagued by background auto-assembly, unstable molecular interactions, and/or the formation of a rigid complex lacking dynamic tunability. We have developed a B12-induced, optically tunable protein assembly system by splitting the photoreceptor protein, CarHC. The presence of a cobalamin cofactor, either AdoB12 or MeB12, is essential for the rapid reassembly of this system, which, on light exposure, can further form a remarkably stable protein adduct (Figure 6). This tightly controlled, inducible protein assembly method has enabled the creation of diverse smart and cyto-compatible hydrogels. In addition to the opportunities brought for materials science and molecular engineering, this new protein chemistry has enriched the toolbox of GECC. This new GECC, with its marked efficiency, specificity and photoresponsiveness, might also provide an alternative to the existing optogenetic tools for controlling biological signaling in vivo. This study has been published in Science Advances (Yang et al., Science Advances 2022, 8, eabm5482).
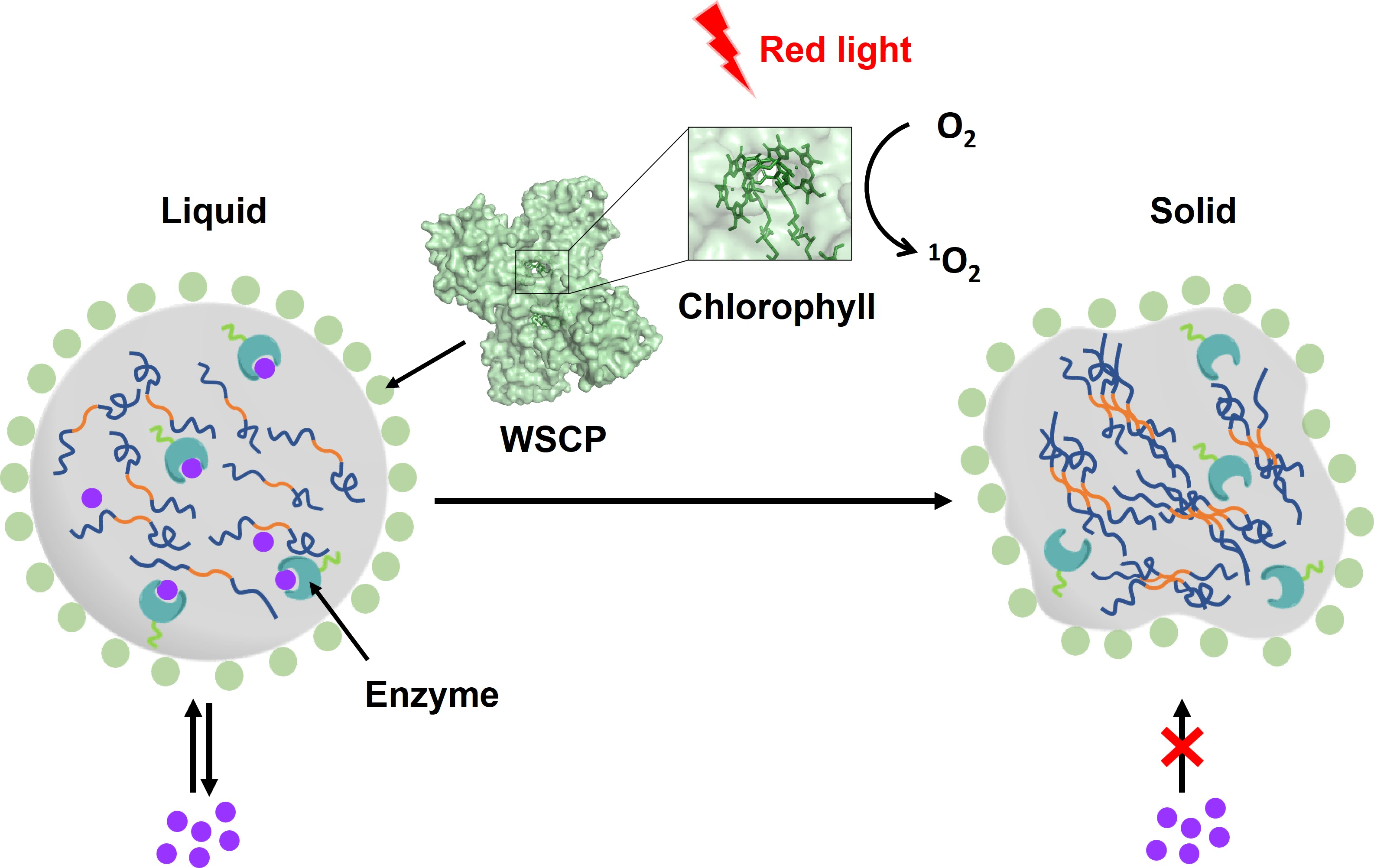
Optogenetic Control over Intracellular Protein Phase Transition
Protein phase transition (PPS) has recently ushered in a paradigm shift not only in our fundamental understanding of biology but also in our approach to biological engineering. However, our inability to precisely control PPS has hindered the research on its roles in biology, as well as its translational applications. Drawing on some naturally occurring photoreceptors such as cobalamins (vitamin B12) or chlorophylls, which are prevalent in nature yet rarely used in biotechnologies, we have developed new optogenetic tools for controlling intracellular protein phase transition. Prompted by our previous success of synthesizing photoresponsive hydrogels using CarHC, we utilized this cobalamin-dependent photoreceptor for controlling, reversibly, the phase transition of various proteins inside cells, including the Shank SAM domains of the postsynaptic density, which furnished us with a powerful tool for studying the roles of PPS in synapse regulation (unpublished results).
Given the lack of long-wavelength-light responsive optogenetic tools, which would otherwise greatly benefit in vivo applications, we adopted the water-soluble chlorophyll protein (WSCP) derived from plants and created an in cellulo protein phase separation system, of which biological activities, including catalysis, can be controlled via a red-light-induced liquid-to-solid phase transition (Figure 7). Central to this is the use of WSCP ─ a remarkably stable, red-light-responsive singlet oxygen generator ─ to decorate the protein condensates. Moreover, these condensates, which retain the light-induced phase-transition behavior in living cells, exhibit marked membrane localization, reminiscent of the semi-membrane-bound compartments like postsynaptic densities in nervous systems. Our system provides a new approach to controllable synthetic membraneless organelles and, alongside its light-induced phase transition, may well serve to recapitulate and interrogate the aging process at the subcellular or even molecular level. This study has been published in Nature Communications (Li et al. Nat. Comm. 2022, 13, 1-13).
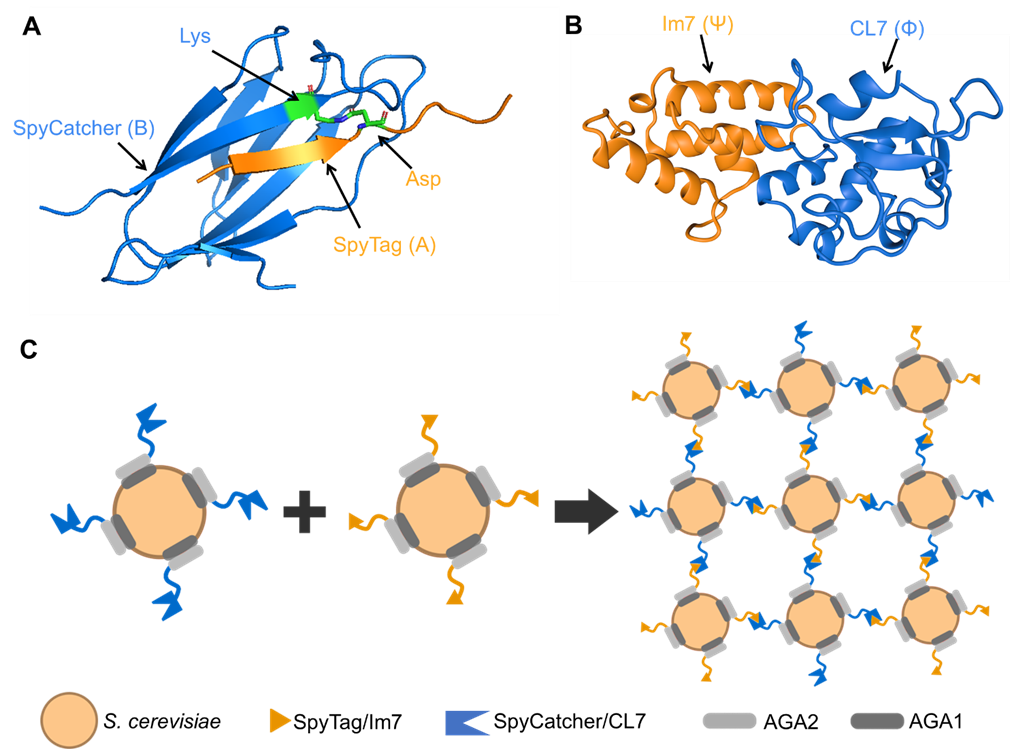
Assembling Single Cells into Multicellular Living Materials for Uranium Extraction
Engineered living materials (ELMs) are gaining traction among synthetic biologists, as their emergent properties and non-equilibrium thermodynamics make them drastically different from traditional materials. However, the aspiration to directly use living cells as building blocks to create higher-order structures or materials, while avoiding chemical modification, remains elusive. Recently we managed to assemble engineered Saccharomyces cerevisiae into self-propagating ELMs via ultra-high-affinity protein/protein interactions. The yeast cells have been genetically engineered to display on their surfaces the protein pairs, SpyTag/SpyCatcher or CL7/Im7, which enable their assembly into multicellular structures capable of further growth and proliferation. The assembly process can be controlled precisely via optical tweezers or microfluidics. This strategy, with its genetic programmability and versatility, can be extended to other engineerable microoganisms, prokaryotic and eukaryotic, thus promising new opportunities for materials synthetic biology.
One immediate implication of this study is the possibility of using ELMs for uranium extraction from seawater. Despite an enormous uranium reserve in the ocean, 1000 times that on land, large-scale extraction of oceanic uranium (uranyl, UO22+) is extremely challenging due to a very low concentration (~ 14 nM) and the presence of excessive competing ions such as calcium, magnesium and carbonate in seawater. Practical, low-cost extraction methods for oceanic uranium mining have yet to be developed. As proof of concept, we incorporated the super uranyl binding protein (SUP) via genetic programming, which rendered these materials suitable for uranium extraction, pointing to their potential for cost-effective chemical separation. This study has been published in Science Advances (Yi et al., Sci. Adv. 2022).
References
1. Sun F & Zhang WB (2017) Unleashing chemical power from protein sequence space toward genetically encoded "click" chemistry. Chinese Chem Lett 28(11):2078-2084.
2. Zhang WB, Sun F, Tirrell DA, & Arnold FH (2013) Controlling Macromolecular Topology with Genetically Encoded SpyTag-SpyCatcher Chemistry. J Am Chem Soc 135(37):13988-13997.
3. Sun F, Zhang WB, Mahdavi A, Arnold FH, & Tirrell DA (2014) Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry. Proc. Natl. Acad. Sci. USA 111(31):11269-11274.
4. Yang ZG, Kou SZ, Wei X, Zhang FJ, Li F, Wang XW, Lin Y, Wan C, Zhang WB, & Sun F (2018) Genetically Programming Stress-Relaxation Behavior in Entirely Protein-Based Molecular Networks. Acs Macro Lett 7(12):1468-1474.
5. Wang R, Yang Z, Luo J, Hsing IM, & Sun F (2017) B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release. Proc. Natl. Acad. Sci. USA 114(23):5912-5917.
6. Kutta RJ, Hardman SJ, Johannissen LO, Bellina B, Messiha HL, Ortiz-Guerrero JM, Elias-Arnanz M, Padmanabhan S, Barran P, Scrutton NS, & Jones AR (2015) The photochemical mechanism of a B12-dependent photoreceptor protein. Nat. Commun. 6:7907.
7. Jost M, Fernandez-Zapata J, Polanco MC, Ortiz-Guerrero JM, Chen PY, Kang G, Padmanabhan S, Elias-Arnanz M, & Drennan CL (2015) Structural basis for gene regulation by a B12-dependent photoreceptor. Nature 526(7574):536-541.
8. Ortiz-Guerrero JM, Polanco MC, Murillo FJ, Padmanabhan S, & Elias-Arnanz M (2011) Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA 108(18):7565-7570.
9. Jost M, Simpson JH, & Drennan CL (2015) The Transcription Factor CarH Safeguards Use of Adenosylcobalamin as a Light Sensor by Altering the Photolysis Products. Biochemistry-Us 54(21):3231-3234.
10. Wei J, Wu W-H, Wang R, Yang Z, Sun F, & Zhang W-B (2018) B12-Dependent Protein Oligomerization Facilitates Layer-by-Layer Growth of Photo/Thermal Responsive Nanofilms. Acs Macro Lett 7(5):514-518.
11. Cao Y, Wei X, Lin Y, & Sun F (2019) Synthesis of bio-inspired viscoelastic molecular networks by metal-induced protein assembly. Molecular Systems Design & Engineering.
12. Kou SZ, Yang X, Yang ZG, Liu XT, Wegner SV, & Sun F (2019) Cobalt-Cross-Linked, Redox-Responsive Spy Network Protein Hydrogels. Acs Macro Lett 8(7):773-778.
13. Luo J, Liu X, Yang Z, & Sun F (2018) Synthesis of Entirely Protein-Based Hydrogels by Enzymatic Oxidation Enabling Water-Resistant Bioadhesion and Stem Cell Encapsulation. ACS Applied Bio Materials 1(5):1735-1740.
14. Park BM, Luo JR, & Sun F (2019) Enzymatic assembly of adhesive molecular networks with sequence-dependent mechanical properties inspired by mussel foot proteins. Polym Chem-Uk 10(7):823-826.
15. Kou S, Yang Z, & Sun F (2017) Protein Hydrogel Microbeads for Selective Uranium Mining from Seawater. ACS Appl Mater Interfaces 9(3):2035-2039.
16. Kou SZ, Yang ZG, Luo JR, & Sun F (2017) Entirely recombinant protein-based hydrogels for selective heavy metal sequestration. Polym Chem-Uk 8(39):6158-6164.
17. Zeng ML, Shang Y, Araki Y, Guo TF, Huganir RL, & Zhang MJ (2016) Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 166(5):1163-1175.
18. Zeng M, Chen X, Guan D, Xu J, Wu H, Tong P, & Zhang M (2018) Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell.